Introduction: The chronic lymphocytic leukemia (CLL) treatment landscape is rapidly evolving, and real-world (rw) outcomes of patients receiving 2L+ therapy in the current treatment paradigm are incompletely understood. This study examined the rw outcomes of patients with CLL/ small lymphocytic lymphoma (SLL) receiving 2 or more lines of therapy (LOTs) using a large, contemporary rw dataset.
Methods: Patients meeting the study criteria were identified in the COTA real-world, clinical database: Aged ≥ 18 years at diagnosis with CLL/SLL who initiated 2L therapy between January 1, 2014 and June 30, 2021. The initial index date for the study was the date of 2L initiation and was updated to the start of each subsequent LOT for LOT-specific analyses. The following time to event outcomes were evaluated for the study population and by LOT using the Kaplan-Meier method: time to next treatment (TTNT: time from index to the initiation of the next LOT or death), time to treatment discontinuation (TTD: time from index to treatment discontinuation for any reason, including end of therapy as prescribed/indicated), rw duration of response (rwDOR: time from the first clinician-documented CR or PR to the first assessment of progressive disease or the initiation of next LOT among responders), rw progression-free survival (rwPFS), and rw overall survival (rwOS). For all time-to-event endpoints, patients were censored at the date of last visit. Rw overall response rate (rwORR) was the proportion of patients who had a clinician-documented PR or CR as the best response to a LOT among all patients who received that LOT.
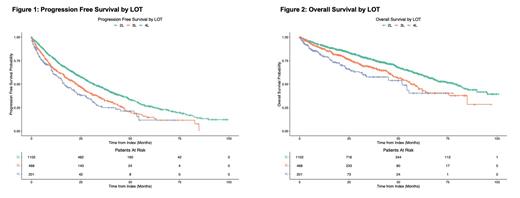
Results: A population of 1,102 patients was identified from the COTA database meeting the study criteria. Key patient and clinical characteristics included: median age at diagnosis of 64 years, 60.9% male, 88.1% treated in a community setting, 74.1% diagnosed in 2014 or earlier, 9.3% positive for TP53 mutation (55.0% unknown), and 7.8% with del(17p) mutation (66.2% unknown). Median TTNT among patients who received 2L (n=1102) was 29.3 months (95% CI: 27.3, 32.6), and median DOR to 2L (n=863) was 31.9 months (95% CI: 27.5, 36.4). Generally, median TTNT and DOR decreased across LOT 2-4. Median TTNT from 3L (n=468) and 4L (n=201) initiation were 21.4 (95% CI: 18.2, 25.1) and 15.1 months (95% CI: 11.5, 20.2), respectively, and median DOR among 3L (n=333) and 4L (n=130) responders were 22.6 (95% CI: 18.3, 26.2) and 15.1 months (95% CI: 11.7, 22.9), respectively. More than three-quarters (78.3%) of 2L patients experienced a physician-documented CR or PR, and rwORR decreased over subsequent LOTs (71.2% in 3L and 64.7% in 4L).
Median TTD varied by treatment type. Patients who received continuous therapy with Bruton's Tyrosine Kinase inhibitors (BTKis) experienced longer median TTD, while patients who received fixed-duration therapies experienced shorter median TTD. Median TTD among patients who received ibrutinib (n=391), acalabrutinib (n=54), or venetoclax + rituximab (n=48) in 2L was 24.8, 32.6, or 23.5 months, respectively, while median TTD among patients who received BR (n=194) or rituximab monotherapy (n=67) was 4.0 or 0.9 months, respectively.
Finally, median rwPFS and rwOS decreased by LOT for patients who received 2L-4L. Median rwPFS for 2L, 3L, and 4L were 31.4 (95% CI: 28.6, 35.5), 22.8 (95% CI: 19.1, 26.4), and 16.5 (95% CI: 13.6, 22.6) months, respectively, and median rwOS were 79.0 (95% CI: 68.9, 85.3), 56.3 (95% CI: 51.6, 64.5), and 51.9 months (95% CI: 33.8, Not Reached), respectively.
Conclusions: In this real-world, contemporary database analysis, median TTNT and DOR exceeded 2 years among 2L patients. Although the adoption of novel therapies has changed the CLL/SLL treatment paradigm and patients who received 2L BTKi therapy experienced numerically greater TTD relative to those who received other treatment types, patients with relapsed/refractory (R/R) disease continue to experience poor rwPFS and rwOS relative to patients in clinical trials. This study demonstrates the continued unmet need for this rw patient population and highlights the need for continued innovation, namely new therapeutic agents leveraging novel mechanisms of action, to improve rw outcomes for patients with R/R CLL/SLL.
Disclosures
Davids:Research to Practice: Consultancy; Secura Bio: Consultancy; TG Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Research Funding; Surface Oncology: Research Funding; MEI Pharma: Research Funding; Mingsight Pharmaceuticals: Consultancy; Merck: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Eli Lilly: Consultancy; BMS: Consultancy; Curio Science: Consultancy; AstraZeneca: Consultancy, Research Funding; Adaptive Biosciences: Consultancy; Aptitude Health: Consultancy; ONO Pharmaceuticals: Consultancy; Ascentage Pharma: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; BeiGene: Consultancy. De Nigris:Merck Sharp & Dohme LLC (a subsidiary of Merck & Co): Current Employment; Merck & Co., Inc.: Current equity holder in publicly-traded company. Prescott:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Leng:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Gandra:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Zettler:C OTA: Current Employment. Fernandes:COTA: Current Employment. Wang:COTA: Current Employment, Current equity holder in private company. Shadman:Genentech: Consultancy, Research Funding; Regeneron: Consultancy; Genmab: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; ADC therapeutics: Consultancy; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Eli Lilly: Consultancy; Janssen: Consultancy; Vincerx: Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; MEI Pharma: Consultancy; AbbVie: Consultancy, Research Funding; Fate Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy; TG Therapeutics: Research Funding; Mustang Bio: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal